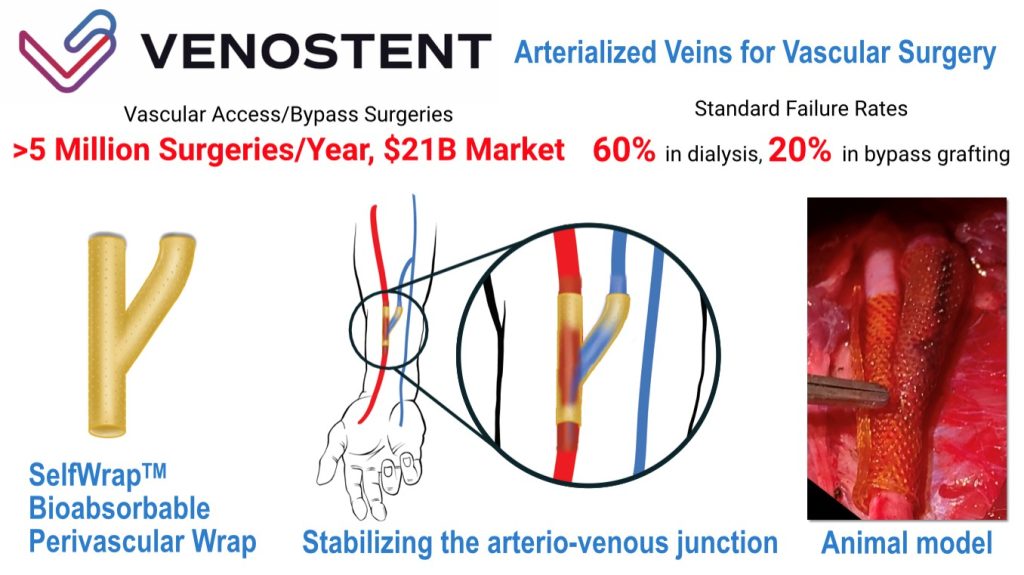
VenoStent, Inc., a Houston, TX-based clinical-stage, venture-backed, therapeutic medical device company developing bioabsorbable wraps to improve outcomes for hemodialysis patients, completed a $16M Series A financing.
The round was led by Good Growth Capital and IAG Capital Partners, with participation from TMC Venture Fund, SNR, Baylor Angel Network / Affinity Fund, Creative Ventures, Cowtown Angels, Alumni Ventures, and other angel investors.
The company intends to use the funds to expand their US Pivotal Trial and their manufacturing capabilities, aiming towards FDA Approval of their novel therapeutic medical device.
Led by CEO Tim Boire, Ph.D., VenoStent is a therapeutic medical device company developing a bioabsorbable wrap, SelfWrap, to transform outcomes in vascular surgery, starting with hemodialysis access. The device is intended to improve the usability and durability of arteriovenous fistulas (AVFs), the artery-vein connections that are surgically created in the arms of chronic kidney disease patients to enable life-saving dialysis treatments.
The company, which has received funding in the past from KidneyX, National Science Foundation (NSF), National Institute of Health (NIH), Y Combinator, Health Wildcatters, and Texas Halo Fund, also has announced that they have been granted Investigational Device Exemption (IDE) from the FDA for their US clinical trial, SAVE-FistulaS.
FinSMEs
01/08/2023